How it Works
Avalanche delivers its therapeutic treatments using an adeno-associated virus (AAV) as a vector to deliver and express, or transduce, a functional gene to the cells of the eye to promote continuous protein production.
To create a safe therapeutic vector, viral genes are removed from AAV and replaced with specific genes encoding a therapeutic protein that potentially treats a variety of ophthalmic diseases. AAV can infect a variety of retinal cell types and remain stable, resulting in long-term therapeutic protein expression. In clinical studies, AAV appears to be safe and well-tolerated when injected into the retina.
Additional Information on How it Works:
 |
 |
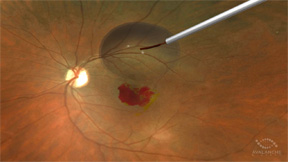
| AVA-101 is injected into the eye in a short surgical procedure. | |
 |
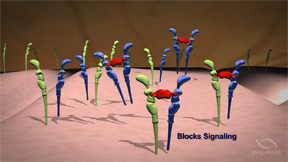 |
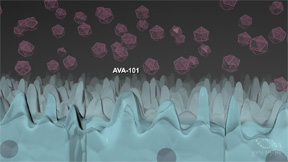
| AVA-101 creates an Ocular BioFactoryTM that is designed to secrete therapeutic protein. The protein in turn blocks VEGF signaling, treating the disease. | |